University of Queensland researchers ready to face next challenge

While The University of Queensland (UQ) COVID-19 vaccine won’t be rolled out to fight this global pandemic, the University’s researchers have made remarkable progress, and they are confident their powerful vaccine platform will be ready for when the world faces another health crisis.
Development of the UQ COVID-19 vaccine (UQ-CSL V451) was halted in December 2020, after Phase 1 trial participants generated a low-level antibody response to fragments of a protein – gp41 – used in the ‘molecular clamp’ technology.
The antibody response was enough to contribute to diagnostic interference with some HIV screening tests.
As the team reflects on the news that its vaccine candidate will not progress to the next phase of human trials, they are taking heart from knowing that the methods they developed in their 11-month quest could be well suited for use in future campaigns against other novel viruses.
That hope rests on the innovative ‘molecular clamp’ approach that the researchers had earlier developed and were relying on.
All the signs had been promising. UQ, the Coalition for Epidemic Preparedness Innovations (CEPI) and biotech company CSL entered into a landmark agreement in June 2020 to manufacture and develop the vaccine.
Clinical human trials began in July 2020 to assess safety and the immune response generated in 216 healthy volunteers. Dosing had been completed, and the data showed the vaccine was eliciting a robust response to the virus.

A member of UQ’s COVID-19 vaccine team in their lab at The University of Queensland. Photography by Glenn Hunt.
A member of UQ’s COVID-19 vaccine team in their lab at The University of Queensland. Photography by Glenn Hunt.
Importantly, there were no observed safety concerns.
But among the reams of positive data from the Phase 1 trial, the unexpected antibody response meant the vaccine could not progress.
UQ vaccine project director Professor Trent Munro, from UQ’s Australian Institute for Bioengineering and Nanotechnology, said trial participants had been fully informed of the theoretical possibility of a partial immune response to this component of the vaccine when they consented to participate in the trial.
“We understood there was the risk of this response, but we knew this was not a safety issue for participants if it occurred,” Professor Munro said.
“As the data began to come in and we analyzed what this meant for the vaccine, we ordered additional tests. To be completely clear, there was no possibility the vaccine could cause HIV infection – and routine follow-up tests confirmed a negative result.”
The gp41 protein, found in the HIV virus, was used to form the ‘molecular clamp’ for the COVID-19 vaccine. The clamp technology, developed by the UQ scientists, stabilizes viral ‘spike’ proteins so that vaccines can be more effective and scaled up rapidly for mass manufacturing.
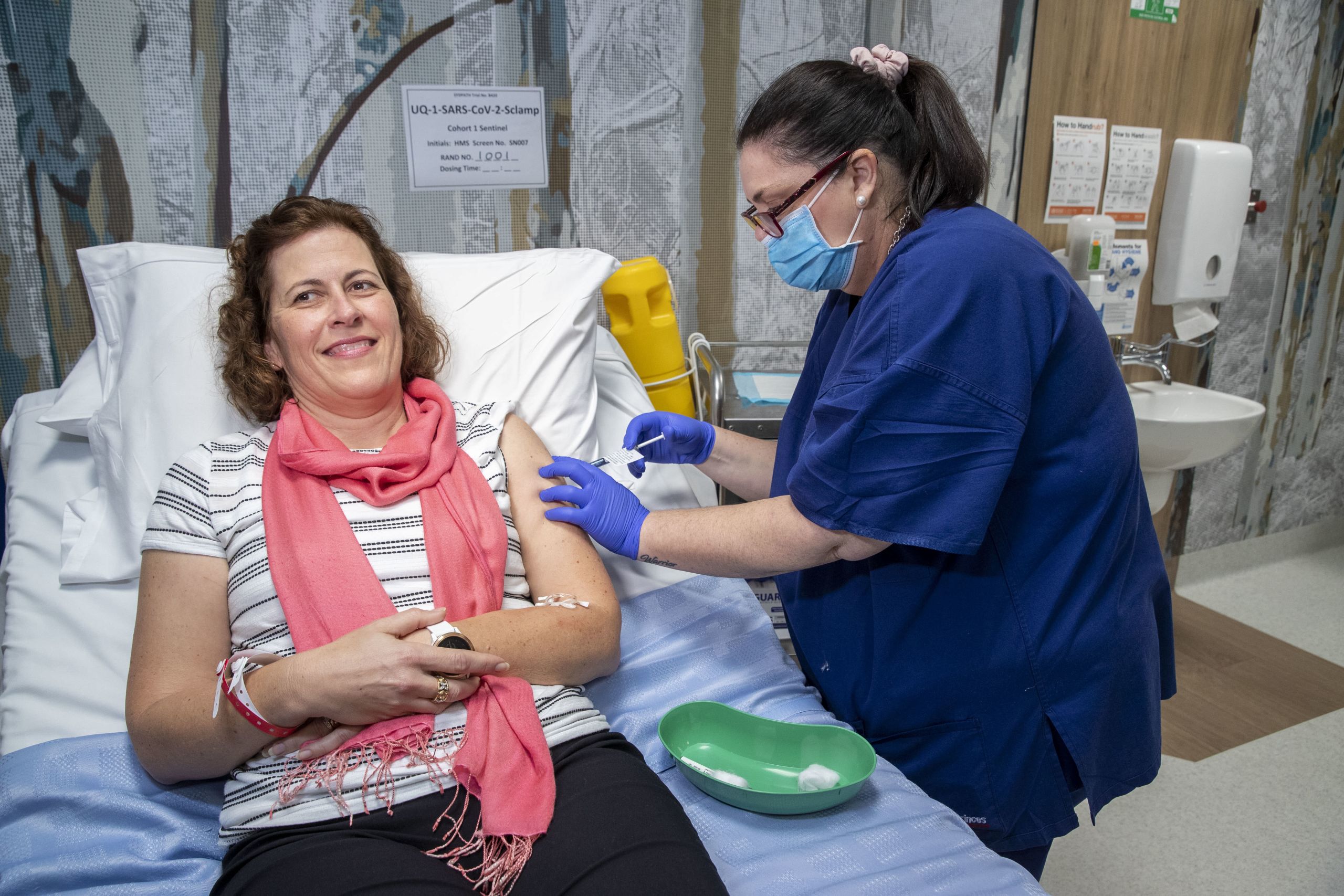
A Queensland patient receives the first dose of UQ’s COVID-19 vaccine on July 13, 2020 during the Phase 1 clinical trials. Photography by Glenn Hunt.
A Queensland patient receives the first dose of UQ’s COVID-19 vaccine on July 13, 2020 during the Phase 1 clinical trials. Photography by Glenn Hunt.
UQ vaccine co-leader and head of the University’s School of Chemistry and Molecular Biosciences, Professor Paul Young, said the gp41 fragment was the most promising of all the proteins he and his colleagues had assessed before the pandemic for that vital stabilizing role.
“The protein pieces that make up the clamp are completely harmless, and adding them to the spike protein provided the required enhanced stability,” Professor Young explained.
“Our intention was always to explore additional options through the course of our research program.”
But that could not be done in time to advance their COVID-19 vaccine candidate.
“If we had looked for another protein rather than proceed with gp41, there would have been a minimum six- to 12-month delay — and we would not have had the same level of confidence that it was even going to work."
“Of all the options, it had provided the greatest stability in those earlier studies, and we engineered it in such a way that we removed the major antibody binding sites from the protein to minimize the impact of the potential immune response.”
Promising findings about the vaccine candidate, up to that point, made the decision to halt the vaccine progress particularly disappointing.
“The hardest thing is that from a safety-data and immunogenicity point of view, including a strong immune response in the elderly, everything looked stellar,” Professor Munro said.
But the HIV testing area is very complicated and, according to Professor Munro, it’s what makes this a very difficult issue to solve.
“The immune response to our vaccine has been shown to interfere with several screening HIV tests – indicating a false positive – which in Australia would be followed up with a further diagnostic test,” he said.
“In the weeks following the initial data, we worked with CSL, the Australian Government advisory committees, HIV experts and pathology experts to understand the impact."
“Any tests that do not give someone clarity can create concern. And, fundamentally, anything that interferes with public confidence in vaccines has to be part of the consideration."
“That’s why we understood the decision to halt our vaccine.”

Queensland’s Premier, Annastacia Palaszczuk, attends the first injections of the Phase 1 clinical trial of UQ’s COVID-19 vaccine on 13 July 2020. Photography by Glenn Hunt.
Queensland’s Premier, Annastacia Palaszczuk, attends the first injections of the Phase 1 clinical trial of UQ’s COVID-19 vaccine on 13 July 2020. Photography by Glenn Hunt.
A promising start
In January 2020, the UQ team accepted a request and funding from CEPI to be one of the first three projects around the world – and the only one in Australia – to apply a rapid-response vaccine platform for COVID-19.
CEPI – a global partnership based in Norway and the UK, and launched in 2017 – funds and supports projects that create and test medical responses while also readying them for manufacture and distribution. CEPI had formed a partnership with UQ in January 2019, designed to advance the molecular clamp technology as a rapid response vaccine pipeline. At that point, the plan was to deploy the technology against such viruses as influenza and Middle East respiratory syndrome, or MERS.
In June 2020, UQ and CEPI entered into an agreement with CSL, which could guarantee the rapid manufacturing of the COVID-19 vaccine, if it proved viable.
The process for developing vaccines typically includes antigen design, animal studies for safety and efficacy, human clinical trials, and preparation for large-scale manufacturing. Those stages typically take years. CEPI’s goal is to identify and support vaccine-development projects that seem likely to accelerate that timeline. One way to do that is to develop the mass-manufacturing process for candidate vaccines at the same time as pre-clinical and clinical trials of efficacy and safety are conducted.
That dual approach was at the core of the UQ project. The scientists worked day and night with colleagues at several research labs and biotechnology companies, trying to find an urgent solution to the pandemic.
In animal models the UQ vaccine candidate generated a strong antibody response, “as good as or better than anyone who has caught, and fought off, COVID-19,” Professor Young said.

UQ’s COVID-19 vaccine vials in the lab at The University of Queensland. Photography by Glenn Hunt.
UQ’s COVID-19 vaccine vials in the lab at The University of Queensland. Photography by Glenn Hunt.
Just as important, the scientists demonstrated that it could be mass produced.
With Phase 1 human trials progressing in July 2020 – and expanding to include older volunteers over the age of 55 – the team was on track to seek regulatory approval to proceed to larger-scale human trials by the end of the year. A project partner in Australia, Seqirus, the influenza vaccine subsidiary of CSL, was set to conduct those trials. The extensive participation of CSL, which produces medicines for such conditions as hemophilia, immune deficiencies and influenza for distribution to more than 70 countries, signaled just how seriously the biotech industry took the Queensland vaccine candidate.
CEPI, the Australian and Queensland governments, and many donors provided funding.
Partners came from far afield. The Queensland scientists conducted pre-clinical testing with colleagues from the University of Melbourne’s Peter Doherty Institute for Infection and Immunity. Kanta Subbarao, the director of the Institute’s WHO Collaborating Centre for Reference and Research on Influenza, and colleagues found that the UQ vaccine candidate induced high levels of neutralizing antibodies against the live COVID-19 virus in cell culture. She said “similar immune responses with SARS vaccines in animal models were shown to lead to protection from infection.”
Other collaborators included the Australian National University and Australia’s national science agency, the Commonwealth Scientific and Industrial Research Organisation (CSIRO). In July, CSIRO’s advanced biologics production facility in Melbourne helped the UQ scientists and CSL create batches of the vaccine for the first human trials. Cytiva, a global therapeutics company, provided solutions for mass production by CSL.
In late August, project co-leader Keith Chappell, an Associate Professor and principal research fellow at the UQ School of Chemistry and Molecular Biosciences, reported to the International Society for Vaccines data from successful animal trials conducted by Viroclinics-DDL. Viroclinics, a long-standing UQ partner, ran those trials in its biosecurity facilities in the Netherlands.
Seqirus contributed its proven Seqirus MF59® adjuvant, which enhances immune response to an antigen. The resulting UQ-CSL V451 provided protection against virus replication and reduced lung inflammation following exposure to the virus in trials in hamsters.

A member of UQ’s COVID-19 vaccine team working in their lab at The University of Queensland. Photography by Glenn Hunt.
A member of UQ’s COVID-19 vaccine team working in their lab at The University of Queensland. Photography by Glenn Hunt.
These steps advanced the vaccine candidate from simple flasks in the lab to a state-of-the-art bioreactor, while ensuring the cells continued to grow and produce the ‘spike’ protein with all the required properties needed to make a successful vaccine.
UQ's COVID-19 vaccine candidate in a syringe on July 13, 2020 during the first human injections as part of the Phase 1 clinical trials. Photography by Glenn Hunt.
UQ's COVID-19 vaccine candidate in a syringe on July 13, 2020 during the first human injections as part of the Phase 1 clinical trials. Photography by Glenn Hunt.
Knowledge is power
For now, monitoring of the Phase 1 trial participants continues, with further analysis of the data to show how long the antibodies to gp41 persist.
“The level of antibody response against HIV is actually very low, and initial signs are already showing that this response is waning,” Professor Munro said.
“We will continue building on what we have learned about this technology and its ability to produce robust, stable vaccines."
“The project demonstrates the clinical trial process and scientific methods can withstand the pressures of speed, and that’s really important, because one of the big debates has been whether vaccine development is progressing too quickly and cutting corners."

Members of UQ’s COVID-19 vaccine team in their lab at The University of Queensland. Photography by Glenn Hunt.
Members of UQ’s COVID-19 vaccine team in their lab at The University of Queensland. Photography by Glenn Hunt.
“If anything, this year’s result has given us even greater confidence that the underlying approaches will provide the world with an incredibly powerful solution for viral pandemics in the future, as well as a raft of viral targets for which vaccines don’t yet exist."
CEPI backed that optimism, saying in a statement that the molecular clamp concept showed great promise.
“CEPI is committed to continuing to work with UQ to resolve this issue so that the platform can be used to develop vaccines against other diseases in the future.”
Professor Munro said the world was in a far better place now, compared to when UQ started its rapid response COVID-19 vaccine program.
“In the first half of 2020, we had zero vaccines for COVID-19, and scientists were unsure that developing a vaccine against the virus was even possible.
“We’re in the fortunate position now where multiple vaccines have reached late-stage efficacy testing. They are showing very promising data, and are starting to be approved by regulators and rolled out.”

Researchers work around the clock in UQ’s COVID-19 vaccine lab. Photography by Glenn Hunt.
Researchers work around the clock in UQ’s COVID-19 vaccine lab. Photography by Glenn Hunt.
This content was paid for and created by The University of Queensland. The editorial staff of The Chronicle had no role in its preparation. Find out more about paid content.



