A COVID-19 testing solution:
How colleges and universities can get back to business as usual amid the new normal

“There’s an urgent need to simplify testing for COVID-19 so that people who are infected can be easily and quickly identified,” says Richard Head, Director of the Genome Technology Access Center at the McDonnell Genome Institute. Professor Head has first-hand knowledge of COVID-19 testing at Washington University in St. Louis as one of the co-developers of a new saliva test.
COVID-19 testing speed and accessibility have been some of the biggest challenges to re-opening colleges and universities this fall. The ability to identify an infection and contain its spread as schools and businesses open their doors remains in debate for communities working to keep people safe.
Further, each educational institution, as its own cohesive community, is tasked with protecting the health and safety of every member. However, with campus re-openings come increased transmission and growing incidence rates. Researchers at the University of North Carolina recently calculated an increase of 2.4 daily cases per 100,000 people in counties with a campus that opened for in-person instruction. While this seems inevitable as students move into university housing and return to classes, educators must weigh these risks with the rewards of teaching and engaging students.
As staff demand regular access to testing and sports teams push to resume practices and games, ease and accessibility of testing is paramount. By instituting a policy for consistent testing across students, sports teams, faculty and staff, colleges and universities could more readily detect positive cases and move quickly to contain potential outbreaks. Of course, this is easier said than done.


Biotech with a vision
Fluidigm, a biotechnology tools provider, might just have a solution. The company focuses on the most pressing needs in translational and clinical research, including cancer, immunology and immunotherapy. With an emphasis on developing high-throughput tools used to investigate the immune system, Fluidigm technology is primed to support studies on infectious disease.
So much so in fact, that scientists at the McDonnell Genome Institute and the Department of Genetics at Washington University School of Medicine in St. Louis collaborated with Fluidigm to develop a new COVID-19 test. Answering the demand for an easy-to-use, high-capacity testing approach, the team created a high-throughput saliva-based test using Fluidigm microfluidics technology.
The noninvasive saliva test, which can run up to 6,000 samples* in one day on one system, addresses the pressing need for increased testing and for a significant improvement in testing accessibility. The test has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for use by labs certified under the Clinical Laboratory Improvement Amendments (CLIA) in the United States.
“In addition to the attractive sample input, we chose the Fluidigm workflow because it combines extraordinary throughput per system with a robust supply chain from a trusted large-scale supplier. We believe this approach will enhance testing coverage in critical populations,” says Andrew Lukowiak PhD, CEO of San Diego-based Millennium Health.
Easier testing using saliva
The Advanta™ Dx SARS-CoV-2 RT-PCR Assay enables testing through collection of saliva, which is significantly easier than invasive nasopharyngeal swab collection. This approach not only could enhance testing coverage in critical populations but also encourages a higher participation rate in public testing with a more comfortable collection method. Saliva-based testing can be done easily by the patient with supervision of a healthcare provider, thereby negating the protective gear requirements of nasopharyngeal testing.
Recent studies comparing different collection methods for SARS-CoV-2 detection have validated the sensitivity of saliva testing. A clinical study associated with the EUA submission for the test demonstrated 100 percent agreement between the saliva results from the Advanta Dx SARS-CoV-2 RT-PCR Assay and the results from paired nasopharyngeal samples tested with authorized assays.
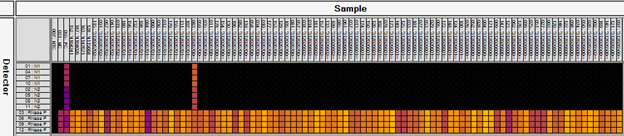
Results from an Advanta Dx SARS-CoV-2 RT-PCR Assay run. The first three columns are controls (no sample, positive control and negative control). All other columns are saliva samples. Clearly, all samples are negative except the one positive shown.
Results from an Advanta Dx SARS-CoV-2 RT-PCR Assay run. The first three columns are controls (no sample, positive control and negative control). All other columns are saliva samples. Clearly, all samples are negative except the one positive shown.
“Rapid, reliable testing that is widely available to the public is essential in combatting the COVID-19 pandemic,” said Jeffrey Milbrandt, MD, PhD, Executive Director of the McDonnell Genome Institute and head of the Department of Genetics at Washington University School of Medicine. “The close collaboration between teams at Washington University and Fluidigm aided our efforts to quickly develop this high-throughput assay for SARS-CoV-2 that relies on a saliva sample. Such a test could help overcome supply chain bottlenecks that have limited testing for COVID-19 and help identify infections.”
The Advanta Dx SARS-CoV-2 RT-PCR test is run on the Fluidigm Biomark™ HD platform, which provides throughput and cost advantages that reduce the impact of capacity‑constrained supply chains. The company’s microfluidics technology enables processing of more samples per batch and uses a fraction of expensive testing reagents per sample as compared to more traditional, microwell plate-based PCR technology typically used for current testing. The Biomark HD platform can generate as many as 6,000* test results per day on a single system.
COVID-19 Campus Safeguard Program improves availability of noninvasive, saliva-based SARS-CoV-2 tests for higher education institutions
Beginning in October, Fluidigm initiated a unique program for colleges and universities, empowering them to test their campuses with the Advanta assay. The COVID-19 Campus Safeguard Program offers easy-to-use saliva tests for students, faculty and staff at an academic rate, supporting a healthy campus by scaling testing for classes, activities and university housing.
Campus communities offer means to satisfy such a diversity of interests. From academic clubs and sports teams to Greek life and advanced research, students and faculty participate in activities and academics that make educational institutions what they are. To dissolve these activities diminishes the value that colleges and universities provide. By managing the current pandemic with saliva testing, institutions can deliver results that enable quick, informed decisions.
“Speed, scale and early detection have been critically important since the beginning of this health crisis, and the addition of improvements in ease of use—eliminating the invasive nasopharyngeal swab protocol without compromising performance—could make this test a game changer for the next phase of the global pandemic response,” says Chris Linthwaite, President and CEO of Fluidigm.
The Fluidigm COVID-19 Safeguard program provides a quick, easy and accessible testing resource that can help keep colleges and universities open. Service providers include Millennium Health, Gnome Diagnostics, Immunogenomics, Neogenomics and Vero Dx.
*Actual results may vary based upon the following factors, including but not limited to, laboratory processes, workflows, equipment and the number of operators.
Intended Use
Advanta™ Dx SARS-CoV-2 RT-PCR Assay is a real-time Reverse Transcription (RT) PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in saliva specimens collected without preservatives in a sterile container from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to Laboratories which are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, and meet requirements to perform high complexity tests.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in saliva specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. Negative results for SARS-CoV-2 RNA from saliva should be confirmed by testing of an alternative specimen type if clinically indicated.
The Advanta™ Dx SARS-CoV-2 RT-PCR Assay is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures. The Advanta™ Dx SARS-CoV-2 RT-PCR Assay is only for use under the Food and Drug Administration's Emergency Use Authorization.
This content was paid for and created by Fluidigm. The editorial staff of The Chronicle had no role in its preparation. Find out more about paid content.



